Adverse Event reporting information can be found in footer
Request a Meeting
Ferring Pharmaceuticals Ltd has been supplying medicines in obstetrics for over 25 years. The current product portfolio has treatments for induction of labour, prevention of postpartum haemorrhage due to uterine atony.
On this website you can find information and educational resources on our products and therapy areas, along with the prescribing information.

Propess is indicated for initiation of cervical ripening in patients at 37 completed weeks of gestation.1
Learn More Prescribing Information
Pabal is indicated for the prevention of postpartum haemorrhage due to uterine atony 2
Prescribing Information
Tractocile is indicated to delay imminent pre-term birth in pregnant adult women with a gestational age from 24 until 33 completed weeks.3
Prescribing Information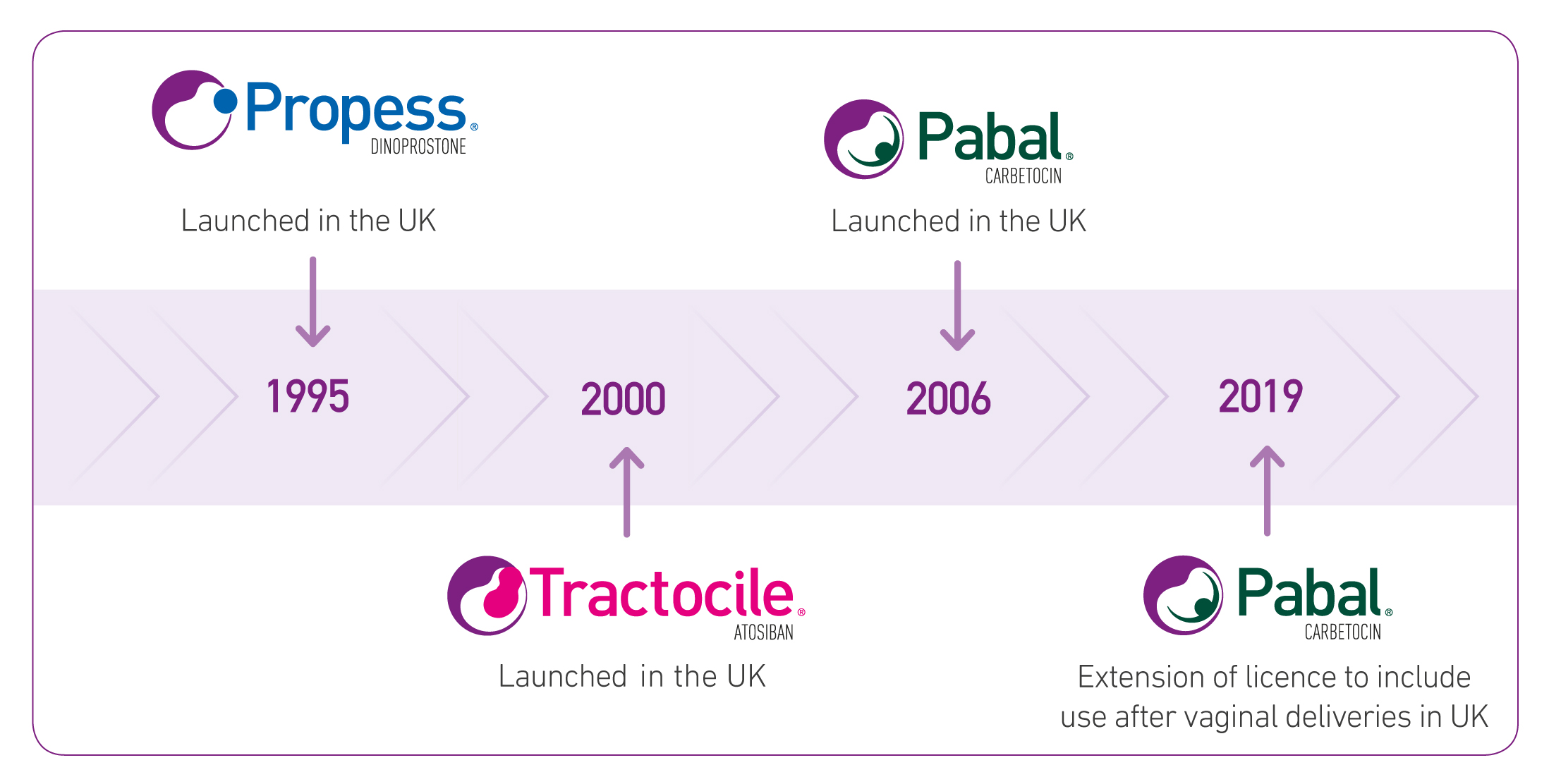
The Ferring Philosophy places people at the heart of what we do. We will address the needs and support those on whom we have an impact in the communities where we operate. Our philosophy and values determine not just what we do, but how we conduct ourselves in the marketplace, with our patients, employees, regulators, business partners and local communities.
We aim to listen with respect and act with integrity.
Making a difference to people’s health and quality of life, today and tomorrow.
Learn MoreJob Code: UK-RMMH-2000023 - Date of preparation: March 2022